Protocol for imaging Adenosin A2A receptors in non-fixed HCT116 colorectal tumor cells
Seed 1 ml of HCT116 cell suspension (5x104 cells/ml of McCoy + 1% Pen/Strep + 10% FBS complete medium) into the inner well of a glass bottom μ-Dish 35 (Ibidi). After cell attachment, add 1 additional ml of complete medium to ensure optimal grow conditions, and incubate them at 37°C and 5% CO2 until confluence.
Prior to cell labelling, remove the complete culture medium and wash once with FBS-free medium. Add 1ml of the Celtarys adenosine receptor fluorescent ligand CELT-316 at a final concentration of 250nM in medium without FBS. CELT-316 selectively binds to Adenosine A2A receptor with a Ki of 116.1nM.
Directly observe the HCT116 live cells on an inverted confocal microscope (Leica SP8), using a 40x oil-immersion objective (HC PL APO CS2 40x/1.30NA) (3% 552 laser beam and Hybrid (HyD3) detector). It is not necessary to wash the cells before visualization; no background was observed with the Celtarys fluorescent ligand. Staining is achieved shortly after addition of CELT-316.
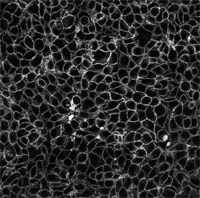
Representative image of HCT116 cells stained with Celt-316. Image was taken 2min after the addition of Celt-316 to the medium.
Protocol for imaging Adenosin A2A-A3 receptors in non-fixed HCT116 colorectal tumor cells
Seed 1 ml of HCT116 cell suspension (5x104 cells/ml of McCoy + 1% Pen/Strep + 10% FBS complete medium) into the inner well of a glass bottom µ-Dish 35 (Ibidi). After cell attachment, add 1 additional ml of complete medium to ensure optimal grow conditions, and incubate them at 37°C and 5% CO2 until confluence.
Prior to cell labelling, remove the complete culture medium and wash once with FBS-free medium. Add 1ml of the Celtarys adenosine receptor fluorescent ligand Celt-327 at a final concentration of 100nM in medium without FBS. For visualization purposes, cells can be co-stained with a green live-cell dye (Calcein).
Directly observe the HCT116 live cells on an inverted confocal microscope (Leica SP8), using a 40x oil-immersion objective (HC PL APO CS2 40x/1.30NA) (3% 552 laser beam and Hybrid (HyD3) detector for Celt-327 and 2% 488 laser beam, PMT 492-562 detector for Calcein). It is not necessary to wash the cells before visualization; no background was observed with the Celtarys fluorescent ligands. Staining is achieved shortly after addition of Celt-327, with fluorescent levels peaking at 5-8mins.
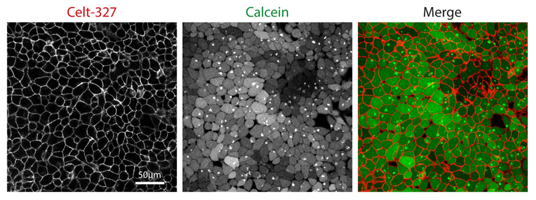
Representative image of HCT116 cells co-stained with Celt-327 and Calcein. Images were taken 2min after the addition of Celt-327 to the medium.
High Content Screening protocol for Cannabinoid Receptors assays
METHODOLOGY:
Cannabinoid CB2 receptor competition binding experiments were carried out in a CellCarrier-96 Ultra Microplate (PerkinElmer 6055302).
40.000 cells per well of a HEK-CB2 cell line were seeded 24 hours prior to assay.
The culture medium was replaced with Hank’s balanced salt solution (HBSS. Sigma H6648). In each well was incubated CELT-331 at a concentration of 30 nM.
The ligand displacement (signal inhibition) was determined in the presence of increasing concentrations of GW405833 (Sigma G1421).
Hanks balanced salt solution was used as assay buffer for all dilutions. The reaction mixture (Vt: 100 μl/well) was incubated at 37°C for 60 min. After washing (100 μl HBSS) the cells were fixed with paraformaldehyde solution 4% in PBS (Chemcruz sc-281692). A cycle of 3 washes was performed with 100 μl of HBSS and Hoechst 33342 trihydrochloride trihydrate (Invitrogen H3570) (dilution 1:2000) was added. A final wash was performed and a 100 μl/well volume of HBSS was added.
Microscopy images were obtained using an Operetta high-content analysis system (PerkinElmer).
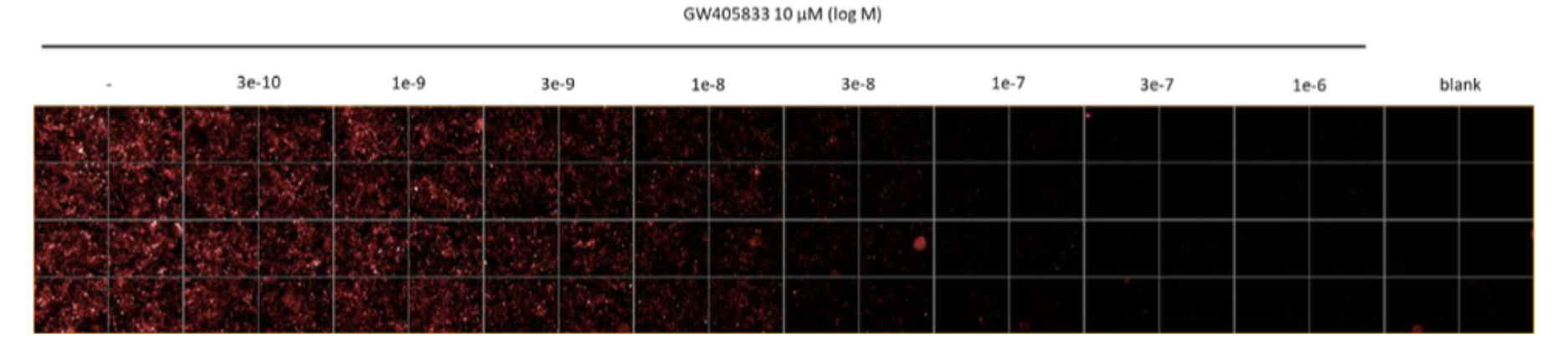
Representative example of the competition binding between CELT-331 30 nM and GW405833.
ASSAY VALIDATION:
The HCS assay was validated using GW405833 as a reference compound binding to Cannabinoid CB2 receptor.
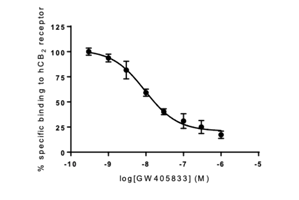
Fig. 1: Representation of the concentration-response curve of GW405833, antagonist for human CB2 receptor, generated from the fluorescence intensity of CELT-331, measured on Operetta high-content analysis system. The mean ± SEM (vertical bars) obtained in duplicate wells by extracting 9 images from each of them is shown.
Table 2: Value (IC50) obtained and described in the literature for the compound used to validate the assay.
| Receptor | Compound | IC50 (nM) obtained | Ki (nM) described | Bibliographic Reference |
| hCB2 | GW405833 | 16.6 | 12 | Brogi et al., Eur J Med Chem 2011; 46:547-555 |
Protocol for competition binding microscopy in transfected cells
Competition binding experiments were carried out in a CellCarrier-96 Ultra Microplate (PerkinElmer 6055302).
30.000 cells per well of a cell line expressing the desired receptor were seeded 24 hours prior to assay.
The culture medium was replaced with Hanks balanced salt solution (HBSS. Sigma H6648). In each well was incubated the compound tested; concentrations of the different compounds were established based on the Ki value obtained in radioligand binding assays.
The ligand displacement (fluorescent signal inhibition) was determined in the presence of a known binder at high concentration (for instance, for A3 receptor competition binding assay, MRS 1220 (Sigma M228) was used). Hanks balanced salt solution was used as assay buffer for all dilutions.
The reaction mixture (Vt: 100 μl/well) was incubated at 37°C for 60 min.
After washing (100 μl HBSS) the cells were fixed with paraformaldehyde solution 4% in PBS (Chemcruz sc-281692). A cycle of 3 washes was performed with 100 μl of HBSS and Hoechst 33342 trihydrochloride trihydrate (Invitrogen H3570) (dilution 1:4000) was added to visualize nuclei.
A final wash was performed and a 100 μl/well volume of HBSS was added.
Microscopy images were obtained using an Operetta high-content analysis system (PerkinElmer).
Representative Examples
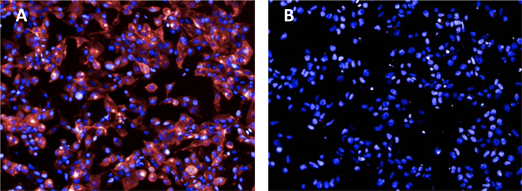
Competition binding between Celtarys fluorescent ligand CELT-171 hA3 Adenosine receptor fluorescent antagonist and MRS 1220 in Hela-A3 cell line.
Hela cells expressing the human A3R labelled with:
A) Celtarys fluorescent ligand CELT-171 hA3 Adenosine receptor fluorescent antagonist at 2 nM (pink) and Hoechst 33342 (blue)
B) CELT-171 hA3 Adenosine receptor fluorescent antagonist at 2 nM and Hoechst 3342 (blue) preincubated with MRS1220 100 nM.
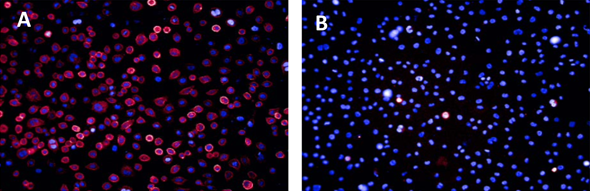
Competition binding between Celtarys fluorescent ligand CELT-331 hCB2 Cannabinoids receptor fluorescent ligand and GW405833 in HEK-CB2R cell line.
HEK cells expressing the human CB2R labelled with:
A) Celtarys fluorescent ligand CELT-331 hCB2 Cannabinoids receptor fluorescent ligand at 30 nM (pink) and Hoechst 33342 (blue)
B) CELT-331 hCB2 Cannabinoids receptor fluorescent ligand at 30 nM and Hoechst 3342 (blue) preincubated with GW405833 10 μM.
Live Imaging protocol for GLP1R fluorescent ligands
Following three different protocols for live imaging of GLP1R taken from Ast et al., 2020 (https://pubmed.ncbi.nlm.nih.gov/31980626/) and Ast et al., 2022 (https://pubmed.ncbi.nlm.nih.gov/35557759/)
Assay 1
CHO-K1 and SNAP-GLP1R:CHO-K1 cells were seeded (60,000 cells/well) on µ-slide 8-well glass bottom dishes (ibidi, 80826) and grown for 2 days at 37 °C in a humidified 5% CO2 incubator.
For imaging, cells were incubated for 30 min at 37 °C in a humidified 5% CO2 incubator in culture medium supplemented with 200 nM LUXendin (either LUXendin645-CELT-112 or LUXendin762-CELT-113) and 5 µM Hoechst33342.
Cells were washed once in cell culture medium and imaged in live cell imaging buffer (Invitrogen, A14291DJ) at 37 °C and 5% CO2 using a Ti-E Nikon epifluorescence microscope equipped with pE4000 (cool LED), Penta Cube (AHF 66-615), 60x oil NA 1.49 (Apo TIRF Nikon) and imaged on SCMOS camera (Prime 95B, Photometrics) operated by NIS Elements (Nikon).
For excitation the following wavelengths were used: LUXendin645-CELT-112: λ = 635 nm; LUXendin762-CELT-113: λ = 740 nm.
Assay 2
For confocal imaging, CHO-K1 and SNAP-GLP1R:CHO-K1 were seeded in 96-well glassbottom plates (Eppendorf, E0030741030) and kept at 37 °C and 5% CO2 until labeling in culture media supplemented with 200 nM LUXendin551-CELT-111 and 500 nM SNAP label at 37 °C, 5% CO2 for 30 min and 4.4 µM Hoechst33342 for 5 min.
After one wash, cells were imaged in culture media using an LSM880 meta-confocal microscope equipped with GaAsP spectral detectors and a 63x water NA 1.20 objective.
For excitation / emission the following wavelengths were used: Hoechst33324: λ = 405 nm / 410-507 nm, LUXendin551-CELT-111 and SBG-TMR: λ = 561 nm / 570-622 nm
INS1 832/3, INS1 832/3 GLP1R-/- and SNAP-GLP1R:INS1 832/3 cells were plated onto Mattek glass bottom dishes the day before imaging, and imaged on a Zeiss LMS780 confocal microscope using a Plan-Apochromat 63x oil 1.40 NA objective for 2 min after addition of 100 nM LUXendin.
Assay 3
Islets were incubated with 100 nM LUXendin551-CELT-111 for 1 h at 37 °C in culture medium. Islets were washed three times and were imaged in culture medium using a Zeiss LSM880 AxioObserver microscope equipped with GaAsP spectral detectors and a 40x water NA 1.2 Korr FCS M27 objective.
For excitation / emission the following wavelengths were used: LUXendin551-CELT-111: λ = 561 nm / 569-667 nm.
Click to download PDFTaken from Ast et al., 2022 (https://pubmed.ncbi.nlm.nih.gov/35557759/)
Non-invasive in vivo imaging protocol forLUXendin762- CELT-113 GLP1R fluorescent ligand
Whole body fluorescence accumulation and distribution was assessed in male athymic nude mice 8 weeks of age using an IVIS Spectral CT (Perkin Elmer).
Mice were anesthetized with inhaled isoflurane and baseline images were acquired. Then, mice were intraperitoneally or subcutaneously injected with 100 μL of saline or 5 μM LUXendin762-CELT-113.
Images were collected using a broad excitation and emission series combination ranging from 640 to 675 nm and 680 to 760 nm, respectively at 30 minutes and 1 hour post injection.
At the end point, animals were sacrificed, and tissues (pancreas, heart, brain, lung, kidney, liver, and spleen) were harvested for ex vivo fluorescent analysis.
Spectral unmixing and quantification were analyzed using Living Image Software.
Two Photon in vivo Imaging for LUXendin551-CELT-111 GLP1R fluorescent ligand
Female NOD/ShiLtJ mice 8 weeks of age were anesthetized with isoflurane. A small, vertical incision was made to expose the intact pancreas. Then, the exposed pancreas was placed on a 50mm glass-bottom dish for imaging on an inverted microscope. Body temperature was maintained using heating pads and heating elements on the objective.
The mouse received, via retro-orbital injection, Hoechst 33342 (1mg/kg in PBS) to label nuclei, albumin-AF647 (1mg/kg in PBS) to label vasculature, and 75μL of 30 µM LUXendin551-CELT-111. Images were collected using a Leica SP8 microscope, equipped with a ×25/0.95 NA objective and Spectra Physics MaiTai DeepSee mulitphoton laser.
Excitation was delivered at λ = 800 nm for Hoechst and Albumin-AF647, with signals collected at λ = 410-500 nm and λ = 550-590 nm, respectively. LUXendin551-CELT-111 was excited at λ = 1050, with signal collected at 650-700 nm.
A conventional PMT was used for Hoechst, with a HyD detector used for Albumin-AF647 and LUXendin551-CELT-111. Blood was collected from the tail vein prior to and 30 min after LUXendin551-CELT-111 injection, and glucose was measured using an AlphaTrak2 glucometer. After imaging, unconscious mice are euthanized by cervical dislocation.
Click to download PDFProtocol for E3 ubiquitin ligase VHL affinity binding assay using TR-FRET
CELT-050, a potent VHL E3 ligase fluorescent ligand, was used as fluorescent probe in a TR-FRET assay to study the affinity of a known inhibitor of E3 ubiquitin ligase VHL, VH298.
Click to download PDF
